Abstract
Background: ADAMTS13 is a metalloprotease that is essential for the cleavage of von Willebrand factor (VWF). Deficiency of plasma ADAMTS13 activity causes a potentially fatal blood disorder, thrombotic thrombocytopenic purpura. Previous studies demonstrated a two-fold increase in the proteolytic activity of ADAMTS13 towards the VWF73 substrate by deleting the distal C-terminal domains, decreasing the pH, or binding a monoclonal antibody to the C-terminal domains. This suggests an allosteric interaction between the distal and proximal domains of ADAMTS13 modulated by pH.
Objectives: To identify peptide regions within a recombinant full-length ADAMTS13 (rADAMTS13) responsible for the pH-modulated allosteric mechanism.
Methods: To identify regions in the rADAMTS13 that undergo conformational changes in response to a change in pH, we performed hydrogen deuterium exchange plus mass spectrometry (HX-MS) at three different pH conditions, pH 6, pH 7, and pH 8. Because the rate of hydrogen and deuterium exchange is dependent upon the pH in a logarithmic fashion, a single time point from each of these pH conditions were compared. For instance, 6000 s was used for pH 6, 600 s for pH 7, and 60 s for pH 8. Any differences in the deuterium incorporation in these conditions in a given peptide should be contributed to conformational changes within the protein effecting accessibility and not simply the rate of hydrogen and deuterium exchange.
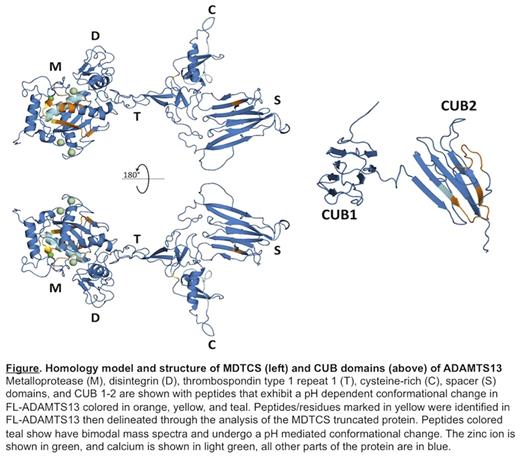
Results: Purified rADAMTS13 and a variant truncated after the spacer domain (rMDTCS) were incubated with deuterium for various times (0- 6000 s). After quenching the reaction by lowering pH to 2.5 and temperature to 0 °C, the rADAMTS13 and rMDTCS were digested by pepsin and fungal protease-13 and then partially resolved by liquid chromatography before being injected into a LTQ ObitrapXL mass spectrometer for peptide identification. Upon comparing the mass spectra of these three conditions, the peptides with mass spectra shift greater than 1 m/z unit as a result of conformational changes from the pH change were identified in the metalloprotease, cysteine-rich, spacer, and CUB-2 domains. In the metalloprotease, three peptide regions were identified (i.e. residues 110 -131, 181-197, and 217-230) with 19, 6, and 1 overlapping peptides, respectively. Within the cysteine rich domain, there were two identified peptide regions, 468 - 480 and 502 - 503. Within the spacer domain, there was only one identified peptide region 629-630, with 5 overlapping peptides. Finally, In CUB-2, there were two peptide regions, 1327-1340 and 1366-1377, with 2 overlapping peptides each (see Figure). All of these regions, with only the exception of residues 217-230, were more accessible to deuterium at pH 6 than at pH 8.
To determine if these results are contingent upon the whole protein being present, and possible intramolecular protein interactions, the HX experiment was repeated using two ADAMTS13 variants (i.e. rMDTCS and rT5C). The rT5C contains the C-terminal TSP1 5-8 repeats and CUB domains. Because of smaller size, more overlapping peptides were recovered, which allows for narrowing down the residues central to the conformational change. Two small areas were narrowed down to several peptides, residues 120-123, with 6 overlapping peptides, and residue 503 within the cysteine rich with 3 overlapping peptides. Curiously, both regions within the CUB-2 domain showed little change with pH, despite 6 overlapping peptides in the residues 1327 -1340 and residues 1366-1377 (see Figure), suggesting the necessity of the proximal domains of the protein to modulate the conformation as a function of pH.
Finally, the regions within the rADAMTS13 that exhibit a pH dependent conformation change are similar to the regions we reported showing bimodal behavior previously. Regions within the metalloprotease, the cysteine rich, and CUB-2 are all shared in residues 217-230, 468-480, and 1327-1328, respectively (see Figure).
Conclusions: Our results, along with the direct binding experiments using a surface plasmon resonance assay, suggest that the N- and C-terminus of ADAMTS13 interact with each other at multiple discrete sites, which mediates the pH-dependent regulation of ADAMTS13 conformation and function.
Zheng: Alexion: Speakers Bureau; Ablynx: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal